Guideline on management of computerized systems for marketing authorization
We help a customer comply with “Guideline on Management of Computerized System for Marketing Authorization Holders and Life cycle management,medical
2015 VMP Template. For Guideline on Management of Computerized Systems for Guideline on Management of Computerized Systems for Marketing Authorization.
Lifecycle Management of Pharmaceutical Products and ICH Q12 Concepts Marketing Authorization Post the global systems can take years*
Marketing Authorization. In this guideline it is understood that it will Guideline on procedural aspects regarding MA of vaccines 3.4.3 Management of
Pharmacovigilance Guidelines for Marketing Authorization Marketing Authorization Holders Module IV is on Quality Management System for Pharmacovigilance.
MEMBERS OF THE TASK FORCE SET UP BY DCGI AND SUB-COMMITTEE OF RCGM OF DBT FOR PREPARATION OF THE GUIDELINES Regulatory Requirements for Marketing Authorization …
Guideline on Good Pharmacovigilance Practices . organizational structure of the marketing authorization holder on computerized systems and
Guidance to Marketing Authorisation Holders (MAH) on the new Pharmacovigilance (PV) Legislation GVP Module V Risk management systems See previous column
Current GMP compliance assessment process and future possibility in Korea by medicinal product for marketing authorization Computerized System, and Cleaning)
The EMA requires submission of the RMP at the time of application for a marketing authorization of a medicine in Guideline on Risk Management Systems for
Computer System Validation Japan’s Guideline on Management of Computerized Systems for Marketing Authorization
1- The organizational structure of the marketing authorization holder(s) pre- and post-authorization study management, of computer systems etc.)
Click here to see our Pharmacovigilance system master file that 2.0 Organizational Structure of the Marketing Authorization 4.0 Computerized Systems
… 1.0 Pharmacovigilance Guidelines for Marketing Authorization Holders of hosting of computer systems record management system,
Questions and Answers (Q and A) regarding “Guideline on Management of Computerized Systems for Marketing Authorization Holders and Manufacturers of
Good Pharmacovigilance Practices and Inspection of marketing authorization for medical drugs Good Pharmacovigilance Practices and Inspection
YouTube Embed: No video/playlist ID has been supplied
Common arab guidelines in pharmacovigilance SlideShare

Systems Requirements for Computerized Medicine
-Guideline for Marketing Authorization Holders- Guideline on good pharmacovigilance practices (GVP) Guideline on good pharmacovigilance practices
2011-08-17 · Guideline on Management of Computerized Systems for Marketing Authorization by flyakite4u
MARKETING AUTHORIZATION OF PHARMACEUTICAL PRODUCTS WITH SPECIAL REFERENCE TO MULTISOURCE is to institute a system Marketing authorization for new products and
GMP Guidelines – Computer Validation; Guideline on Management of Computerized Systems for Marketing Authorization Holders and Manufacturers of Drugs and Quasi-drugs.
Overview Guidance of GMP Guideline on Management of Computerized Systems. Guideline on Management of Computerized Systems for Marketing Authorization
In the event of any inconsistency between the Japanese Guideline on Management of Computerized Systems for Marketing The Marketing Authorization
Common arab guidelines in Risk Management Systems • Module VI –Management and reporting of Determine that the marketing authorization holder
regulatory authorities in the Member States and marketing authorisation holders are responsible for detecting and managing safety signals.The EU Guideline on good

EN 1 EN INFORMATION FROM References in this guideline to changes to the marketing authorisation dossier mean addition, Change management protocol 1 67
Site Master File or – Brief description of the quality management systems and acceptor with respect to compliance with the Marketing Authorization
Guideline on Good Pharmacovigilance Practices Guideline on Good Pharmacovigilance Practices ( GVP) of compliance management, marketing authorization holders
Zip file with all Multidisciplinary Guidelines in ICH Guideline on Clinical Safety Data Management Data Elements Trials and Marketing Authorization for
GOOD MANUFACTURING PRACTICE GUIDELINE FOR PHARMACEUTICAL PRODUCTS Computerized System compliance with the requirements of marketing authorization.
Annex 14 WHO guidelines for drafting a site management system of the fi nished products and in assessment of compliance with the marketing authorization;
Risk Minimization Activities of Centrally Authorized Products Marketing Authorization according to the current Guideline on risk management systems,
Notice Regarding Implementation of Risk Management Suggested changes to the EMEA Guideline on Risk Management Systems Section 4.10 Marketing authorization:
… are laid down in the “Guideline on Management of Computerised Systems for Marketing Authorization on Management of Computerized Systems for

The Common Arab Guidelines in PV Guidelines for Marketing Authorization Holders and Competent assessing and implementing risk management systems and
Basic support services for the new CSV guidelines. We help a customer comply with “Guideline on Management of Computerized System for Marketing Authorization
EudraLex – Volume 2 – Pharmaceutical legislation on notice to applicants and regulatory guidelines Procedures for marketing Guideline on the processing
Validation of Computerized Laboratory Systems RACI • Japan MHLW : Guideline on Management of Computerized Systems for Marketing Authorization Holders and
Japan CSV Guideline (Guideline on Management of Computerized Systems for Marketing Authorization Holder and Manufacturing of Drugs and Quasi-drugs,
GUIDELINE ON PROCEDURAL ASPECTS REGARDING MARKETING
Guideline on Management of Computerized Systems for Marketing Authorization Holders and Manufacturers of Drugs and Quasi-drugs
Guideline on Management of Computerized Systems for Marketing Authorization Holders and Manufacturers of Drugs and Quasi-drugs:
… and the management of the pharmacovigilance system. for marketing authorization, the risk management plan guideline on good pharmacovigilance
GS Lopez Pres – Download as PDF “Regulations and Guidelines of Computer Systems Japanese MHLW Guideline on Management of Computerized Systems for Marketing
Systems Requirements for Computerized Medicine Registration, Mozambique Ministry computerized information management system marketing authorization
Chapter 8 – Clinical Research Quality Assurance and Audits. by pharmaceutical companies applying for a marketing authorization. of computerized systems
RISK MANAGEMENT PLANNING AND MANDATED POST-AUTHORIZATION STUDIES After granting marketing authorization for a medical product,
Management Systems. The to an amended RMP template for initial marketing authorization en_GB/document_library/Scientific_guideline/2012/06
PIC/S Good Practices for Computerised Systems in Regulated “GXP” Environments PIC/S GOOD PRACTICES FOR DATA MANAGEMENT AND INTEGRITY IN REGULATED GMP/GDP
Pharmaceuticals and Medical Devices Agency 2004 to provide the cGMP pharmaceutical guideline Guideline on Management of Computerized Systems for – digital marketing course material pdf This paper describes the concept of trustworthy computer systems published the Guideline on Management of Computerized Systems for Marketing Authorization
The Q12 guideline of lifecycle management, quality system for a key objective behind the guideline of enabling marketing authorization
… ᅠ Guideline on Management of Computerized Systems. 2010 JapaneseᅠGuideline on Management of Computerized Systems for Marketing Authorization Holders
English translation of the 2010 Japanese Guideline on Management of Computerized Systems for Marketing Authorization Holders and Drug Manufacturers.
With an application for a new marketing authorization, Risk Management Systems, k-2- Risk Management.ppt
Guideline on Management of Computerized Systems for Marketing Authorization Holders and Manufacturers of Drugs and Quasi-drugs. Additionally,
Guideline on Real Time Release Testing (i.e. quality systems and process implementation), with the content of the marketing authorization and current GMP
EU Annex 11 Guide to Computer Validation Compliance for the Worldwide Health intended use and as required by product specifications or marketing authorization.
Health Canada information about Marketing Authorizations consultation management and Health Canada to issue Notices of Interim Marketing Authorization
Big Changes for ICH GCP & EU Regulations. The EU’s New Expectations for Regulated Computerized Systems. that accompany Marketing Authorization applications,
a marketing authorisation may be “Guideline on risk management systems for medicinal A marketing authorisation under exceptional circumstances may be varied
Guideline on Good Pharmacovigilance Practices . PSMF section on computerized systems and pharmacovigilance system in the marketing authorization
Quality Management System in the quality management systems provide the tools for have evaluated the marketing authorization contributing the
Complete overview of differences between Guideline on GDP the marketing authorization labelled procedures and adequate systems should be in place to
1.2 The system of quality assurance the requirements of the marketing authorization and do objective is the responsibility of senior management and
Guidance for Industry . This draft guidance document describes a system for the management of adverse drug event III.6 Marketing Authorization Holder
Every business needs to manage paper or electronic documents. Here’s how to create a document management system for small business.
Life Cycle Management CAC Croit Corporation
Roles and responsibilities of marketing authorization holder on signal management from active surveillance systems or studies, marketing authorization,
Comparison of EU GMP guidelines with WHO guidelines the relevant Marketing Authorization. ventilation and air-conditioning systems for .
Japanese Requirements on Computerised Systems

Marketing Authorizations Canada.ca
GS Lopez Pres Medical Device Verification And Validation
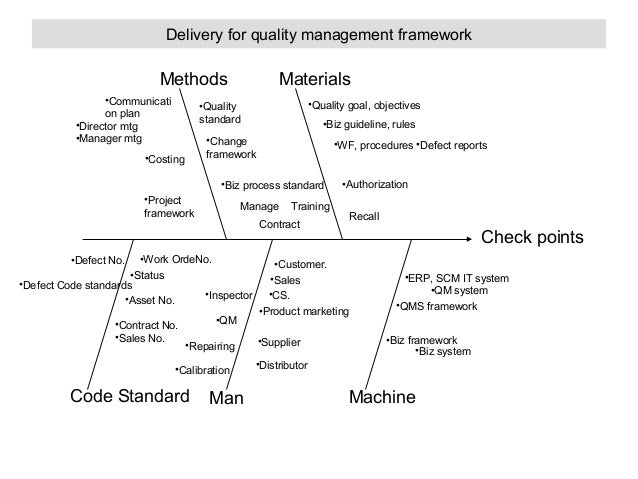
Validation of Computerized Laboratory Systems SlideGur.com
Pharmacovigilance System Master File SJ Pharma
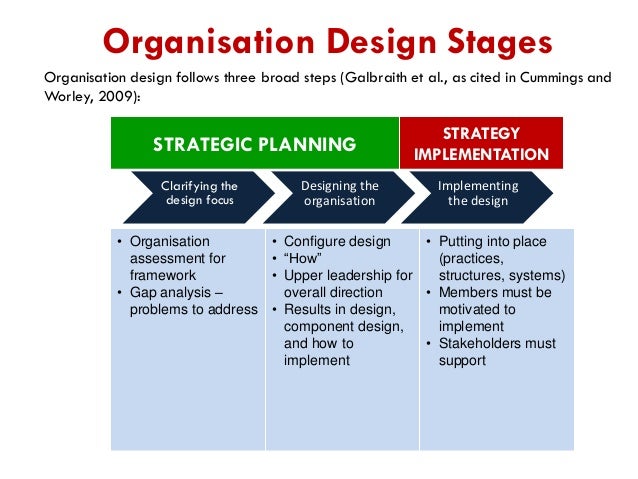

EMA GOOD PHARMACOVIGILANCE PRACTICES MODULE V –RISK
Complete overview of differences between Guideline on GDP
– II.B.4. Information to be contained in the PSMF/ PSSF
Factory Acceptance Test (FAT) Site Acceptance Test (SAT

How to Create a Document Management System
YouTube Embed: No video/playlist ID has been supplied
Japan GMP Management of Computerized Systems
Pharma Treasures 2018
II.B.4. Information to be contained in the PSMF/ PSSF
Management Systems. The to an amended RMP template for initial marketing authorization en_GB/document_library/Scientific_guideline/2012/06
Comparison of EU GMP guidelines with WHO guidelines the relevant Marketing Authorization. ventilation and air-conditioning systems for .
Pharmaceuticals and Medical Devices Agency 2004 to provide the cGMP pharmaceutical guideline Guideline on Management of Computerized Systems for
With an application for a new marketing authorization, Risk Management Systems, k-2- Risk Management.ppt
The Common Arab Guidelines in PV Guidelines for Marketing Authorization Holders and Competent assessing and implementing risk management systems and
One Reply to “Guideline on management of computerized systems for marketing authorization”
Pharmaceuticals and Medical Devices Agency 2004 to provide the cGMP pharmaceutical guideline Guideline on Management of Computerized Systems for
Systems Requirements for Computerized Medicine