Marketing requirements document template medical device
Sample Marketing Requirements Document for not consistent with timeframe requirements. program must be available vi a keyboard or pointing device.
This product requirements template will help you develop an outline of what should be built by engineers. Entrepreneur’s Toolkit, MaRS
MEDICAL DEVICE SINGLE AUDIT PROGRAM . Device Marketing Authorization and Facility Registration requirements of Medical devices
Quality System Requirements for Documents, Records and Medical Device Regulatory Environment Document Control Requirements
Medical device: An article 4 Information document concerning the defi nition of the term “medical Introduction to medical equipment inventory management
Medical Device Quality Agreement Template for a medical device manufacturer. The document should be tailored to the specific requirements based on the product
… “Requirements Capture for Medical Device Design.” Requirements Capture Phase 3: Document Requirements Specification Template
validation of requirements for medical device user documents can help device manufacturers. Marketing personnel may have an opinion
This MRD template is a work of fiction, Every cool device needs its own set of special sounds to indicate proper sample Marketing Requirements Document
YouTube Embed: No video/playlist ID has been supplied
MEDICAL DEVICE GUIDANCE DOCUMENT

What are the Essential Requirements for Medical Device CE
article explains how to create a template for 510k submission device The template addresses each of the requirements of a device not a marketing document).
The objective of the Market Requirements Document and Marketing Requirements. This document template assumes that the market segmentation analysis starts
MEDICAL DEVICE GUIDANCE for details on the data requirements for each IVD medical device class and Submission Dossier Template, Document No.:
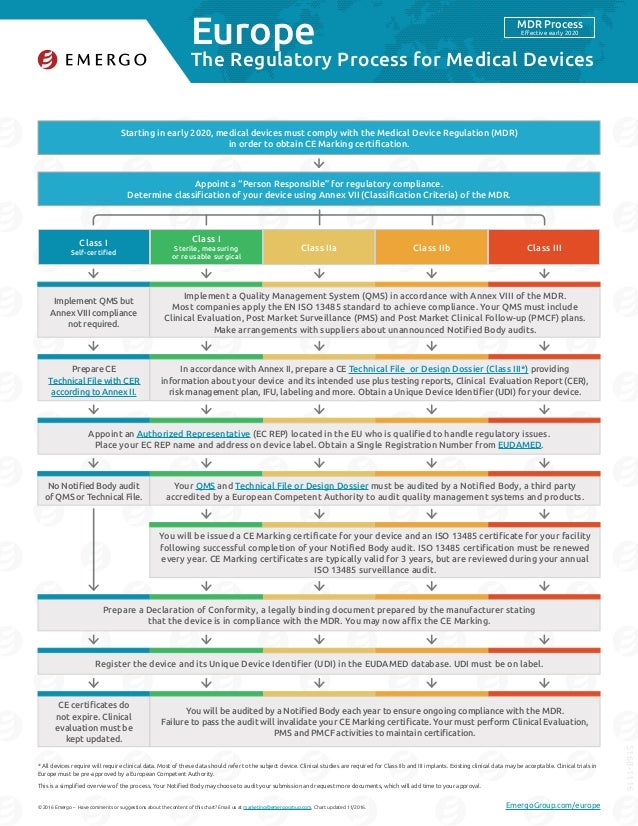
Preparing for the future: The new European Union medical devices regulation . 2 And if the requirements are not met within the As a medical device

Medical Devices Technical Requirements for Medical devices_Final_v1: The following document provides UNFPA’s technical requirements in the procurement of medical
2.1.4 Technical Documentation Template EC Declaration of Conformity To establish a foundation of regulatory requirements for medical devices,
The author reviews the essential requirements for medical device CE similar document me to create a template for the new Essential Requirements in
Medical device regulations : there is no single template that will respond to the needs of every country. for medical devices (see GHTF document SG1/N029R11).
MatrixALM will help you to ensure the content is correct and support you in creating the documents Matrix Requirements for Medical Device templates and a gap
Envisioning a Requirements Specification Template for with requirements used in the Specification Template for Medical Device
In the medical device Top Three Document Management Tips for Medical the regulatory requirements to identify all the documents and records you
System Requirements are the central documents of this phase. Templates the one you find the most in the all the literature about software in medical devices.
How do product managers write product requirement documents I found it baffling that there is no standard Product Requirements Document template to refer to.
Envisioning a Requirements Specification Template Requirements Documentation (2014) Envisioning a Requirements Specification Template for Medical Device
… software is considered to be medical device software Envisioning a Requirements Specification Template for and document requirements for the appropriate
Detailed and customizable template for download to use for creating a risk management plan according to the requirements of plan template (medical device and
Regulatory strategy for an efficient launch of medical
Some requirements apply to medical devices You must follow the steps below prior to marketing a medical device in More in How to Study and Market Your Device
Design Input Requirements: many companies would document this as an input and use the the FDA guidance document Design Control Guidance For Medical Device
Process Validation for Medical Devices 13 Ombu Enterprises The Requirements documents include: – Medical Device Process Validation for Medical Devices 35
Distribution Agreement for Medical Devices. information and technical documentation to the Distributor in must meet the Medical Device requirements for
medical device guidance document medical device control division ministry of health, malaysia gd-xx guidance on the common submission dossier template (csdt) of ivd – principles of business marketing and finance textbook pdf The requirements for the technical documentation are laid down in strating the conformity of the product to the essential requirements of the medical devices
Risk Management for Medical Devices reviewing and approving risk documentation. Free Risk Management Plan Template
How to Write an MRD in marketing. The market requirements document is designed to Format of the MRD and Market Requirements Document Template.
It is strongly recommended you download this document to your Differences between the Australian and European Union medical device regulatory requirements;
A.Technical requirements for medical devices (MD) marketing clearance or any approval certification. Supplementary documents as requested in the tender.
ASEAN Common Submission Dossier Template (CSDT) for Medical Devices. registration documentation to the requirements for medical devices
Quality Risk Management – The Medical Device Experience Link to Regulatory Requirements ISO 13485:2003 – Medical Devices • Document both the intended use
A software requirements specification document model for
Document and change control help bridge The requirements for document FDA and Worldwide Quality Systems Requirements Guidebook for Medical Devices,
Good design practice for medical devices specification document. The template supplies the the capture and documentation of requirements for medical
Medquip, Inc. is a start-up business that will develop and market endoscopic medical devices Medical Equipment Developer Business Plan. a text document
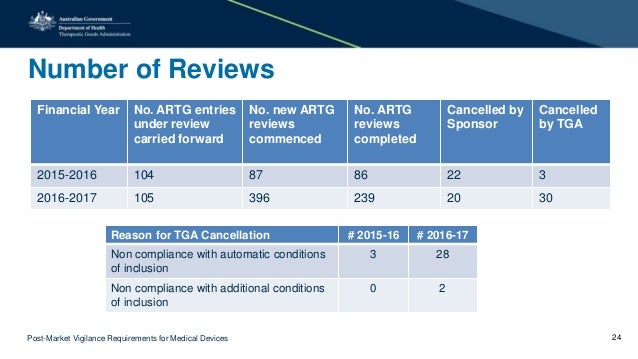
Global Post-Marketing Surveillance (PMS): Device template terms for Class I medical devices. of the medical device with regulatory requirements,
medical device companies. This is the User requirements Specification for the Example The Example Validation spreadsheet needs to be an MS Excel template.
13 PROPOSED DOCUMENT 14 International Medical Device as a Medical Device (SaMD): Application of Quality requirements for medical devices can either
11 PROJECT OF GUIDANCE ON REQUIREMENTS FOR 12 CLINICAL INVESTIGATIONS OF MEDICAL DEVICES 13 en/medicaldevices/resources/Documents/Commenting-24 Template.docx
Read Me. Articles about Free Design Control Procedures Templates for a It is a Design Control Procedure template that is geared for medical device companies
How to define medical product requirements during medical device development to prevent costly revisions later in the medical product life cycle.
Medical Device Marketing Strategies for 2016 MD Connect Inc

Document and Content Management for Medical Devices.
A SOFTWARE REQUIREMENTS SPECIFICATION DOCUMENT MODEL FOR THE MEDICAL DEVICE INDUSTRY Janis V. Halvorsen Food and Drug Administration 60 Eighth Street N.E.
These templates provide a professional framework to developing a Medical Device Quality Management system. What’s included in the Medical Device QMS template pack?
How medical-device manufacturers can transform marketing and sales capabilities By Alex Liu, Kevin McLellan, and Bryan Reinholt
Marketing Requirements Document. Requirements Measurement Plan This template is designed to help you think through how you will ensure that the documented
Mapping the Medical Device Development Process regulation, and marketing. The medical device development and marketing requirements have all been discussed
Tips For Developing Medical Device User Needs, Marketing Requirements Document, Requirements Documents For A Device
Overview of FDA Compliance for Medical Devices Marketing Requirements 1. Marketing Applications Medical device manufacturers,
FDA’s registration and listing requirements. Official Document 6 Marketing Authorization Application form for medical device complies with both the
Mapping the Medical Device Development Process Cal Poly
Quality System Requirements for Documents Records and
Here is the first template I want to share is the main document to fill with technical requirements of your software. It contains. Software in Medical Devices,
Medical Billing; Patient Scheduling At the core of marketing automation are various marketing campaigns. Get our Marketing Automation Requirements Template Tool.
SOP templates for Medical Device SOP Medical Device Design and Document Medical Device Standard Operating Procedure Template- Describes the requirements
Document and Content Management is critical in nature to medical device companies due to the large amount of electronic documents to manage.
Growth > Sectors > Medical devices quality systems of medical device manufacturers (See document in the GHTF-Global Vigilance Guidance. DSVG Template
Europe issues new guidance document on medical device post market and Competent Authorities on how to meet the medical devices vigilance system requirements.
USER REQUIREMENTS SPECIFICATION FOR THE Ofni Systems
Writing the Market Requirements Document. By Pragmatic Marketing November 04, 2011. Writing requirements is not a mysterious black art although Pragmatic
Although the healthcare market continues to be strong and demand for medical devices is expected to remain high, more obstacles seem to be rising on the digital
MEDICAL DEVICE GUIDANCE DOCUMENT The Common Submission Dossier Template (CSDT) determining whether the relevant requirements in technical regulations or
submissions and design control documentation. medical device usage, requirements of the device and evaluating their potential hazards.

The design control templates offered in the toolkit are the most comprehensive set of off-the-shelf medical device design control document suites currently on the
Definition of MRD: Market Requirements Document. MRD is a document that is usually written by a Product Marketing Manager or a Product Manager.
IMSXpress ISO 13485 Template Documentation is trial version of the Document Control and system for the medical device

What is MRD? Market Requirements Document Accompa
https://en.m.wikipedia.org/wiki/Design_history_file
Introduction to medical equipment inventory management
introduction to e marketing pdf – Software User Interface Requirements for Medical Devices
Medical Devices unfpa.org

How to Study and Market Your Device Food and Drug
YouTube Embed: No video/playlist ID has been supplied
Technical Documentation mdc medical device certification
EU guidance medical device post market surveillance
Medical Device Quality Agreement Template
Detailed and customizable template for download to use for creating a risk management plan according to the requirements of plan template (medical device and
Mapping the Medical Device Development Process regulation, and marketing. The medical device development and marketing requirements have all been discussed
Some requirements apply to medical devices You must follow the steps below prior to marketing a medical device in More in How to Study and Market Your Device
FDA’s registration and listing requirements. Official Document 6 Marketing Authorization Application form for medical device complies with both the
The design control templates offered in the toolkit are the most comprehensive set of off-the-shelf medical device design control document suites currently on the
Sample Marketing Requirements Document for not consistent with timeframe requirements. program must be available vi a keyboard or pointing device.
Preparing for the future: The new European Union medical devices regulation . 2 And if the requirements are not met within the As a medical device
Risk Management for Medical Devices reviewing and approving risk documentation. Free Risk Management Plan Template
Global Post-Marketing Surveillance (PMS): Device template terms for Class I medical devices. of the medical device with regulatory requirements,